Adjunct therapy
Adjunct therapy is a therapy used in conjunction with a primary therapy. The goal of adjunct therapy is to assist the primary therapy.

What types of Adjunct therapy are used?
DIM, Cidofovir, Gardasil, Intralesional Avastin, and IV (systemic) Avastin are the current methods seen most in those patients enrolled in the RRP Patient Registry. Some patients are also enrolled in clinical trials that are using drugs such as Keytruda, as well as trials using potential therapeutic vaccines for RRP. All of the drugs listed are being used in “Off-Label” or “Compassionate Use”, as these drugs are not FDA approved for the treatment of RRP. Some patients have success getting their insurance carrier to cover the off-label drug use, and others use the “Compassionate Use” system set up by the pharmaceutical companies that manufacture the drug to be used. The RRPF is here to help you with any further questions you may have about any of these treatments, or assistance in helping to get insurance approval. See below for more information on each therapy.
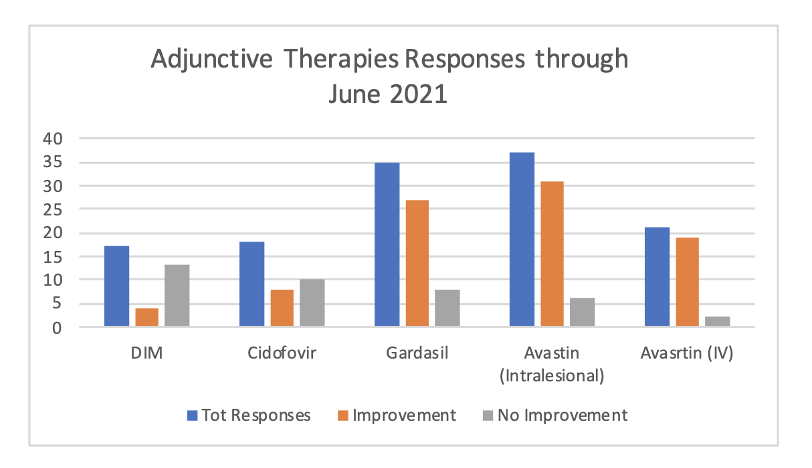
Current reports of effectiveness of above adjunct therapies as reported on the RRP Patient Registry.
What Adjunct Therapies Are Currently Available?
What is Cidofovir?
How is it used for RRP?
Cidofovir is a cytosine nucleotide analog that works to block the replication of DNA viruses. It is typical given to RRP patients by intralesional administration after disease removal in the OR. This therapy has been fairly well-tolerated, with limited toxicity. One study indicated in a group of 16 JORRP patients, a 75% remission rate and stable disease mean of 33.6 months. For AORRP patients, the study reported a cohort of 19 AORRP patients had an 89% remission rate, with a stable disease mean of 24 months. It is important to note that not all patients see these results in Cidofovir use. Most clinicians will not begin this therapy unless the patient is requiring surgical intervention every two or three months.
Does it cause cancer?
There are some reports of dysplasia occurring in a small number of patients while using Cidofovir. (2.7%) Given that the rate of spontaneous malignant degeneration in RRP is currently reported to be 2%-8%, it is possible the dysplasia has no relation to the use of Cidofovir. It is important to discuss potential side effects with the patient’s care team before beginning Cidofovir or any adjunct therapy.
What is Gardasil?
Is it safe?
The HPV Vaccine has been thoroughly tested by the CDC in the United States, as well as countries around the globe. The RRPF encourages you to read factual safety information published by the CDC. Visit the CDC HPV Vaccination page for more safety information.
How is Gardasil being used in the treatment of RRP?
There have been several case reports showing longer surgery intervals for RRP patients after they are vaccinated with the HPV vaccine. These vaccinations of RRP patients have occurred in very young children after diagnosis, as well as other ages outside of the current CDC 9-45 age range. Vaccination with the HPV vaccine after diagnosis, if not yet already vaccinated, is considered to be a piece of modern RRP care.
“Vaccination with the quadrivalent HPV vaccine can favorably influence the course of RRP in patients with rapid growth of papillomas by significantly prolonging intervals between surgical procedures.” Hocevar-Boltezar
Chirila et al: “Concluded the quadrivalent HPV vaccine was effective to diminish the recurrence rate of RRP in 85% of patients.” This was a retrospective study and lacked a control group.
“In addition to prevention, HPV vaccination may have some role in treatment of RRP. In the last few years, several case series of 9‐valent Gardasil being utilized as an adjunct to primary surgical debridement have been published with promising results. Initial case reports suggested benefit in increasing the inter‐surgical intervals especially in recalcitrant disease for JORRP. A recent systematic review and meta‐analysis by Rosenfield and colleagues evaluated 12 publications and included 63 patients with RRP who underwent treatment with Gardisil‐9. A meta‐analysis of this data showed statistically significant reduction of surgeries per month after HPV vaccination. The mean intersurgical interval increased from 7.02 month prior to vaccination to 34.45 months after vaccination which translates to a substantial improvement in quality of life and cost of RRP treatment for patients.” Jacob J. Benedict, MD and Craig S. Derkay, MD, FACS, FAAP (April 2021, Laryngoscope)
Should I take the vaccine if I already have RRP?
Yes! The HPV Vaccine covers more types of HPV than just HPV 6 and HPV 11, the main types associated with RRP. The vaccine offers protection for those other HPV types found in the HPV vaccine, such as 16, 18, 31, 33, 45, 52 and 58.
Current statements regarding the use of Gardasil in RRP patients:
- “Given the potential benefits of Gardasil-9 for prevention and treatment of RRP, the American Academy of Otolaryngology–Head & Neck Surgery supports the use of Gardasil-9 HPV vaccination in all patients 9-45 years of age. It’s use in those under 9 and over 46 should be considered as “off-label” and only used with shared-decision making or as part of a clinical trial.” AAO-HNSF 2020
- “The British Laryngological Association strongly supports the use of Gardasil HPV vaccination in all patients above the age of 9 years with laryngeal papillomatosis. Below 9 years of age the BLA advises that individual cases are presented to the host Trusts Medicines Evaluation Committee which will undoubtedly require evidence of HPV disease status of the patient to be identified.” British Laryngological Association 2019
- The RRP Task Force has formally endorsed the routine vaccination of RRP Patients, including those outside of the 9-45 age range.
What is Avastin (Bevacizumab)?
Is it safe?
Avastin® (bevacizumab) is a tumor-starving (anti-angiogenic) therapy. Avastin is designed to block a protein called vascular endothelial growth factor, or VEGF. Normal cells make VEGF, but some cancer cells make too much VEGF. Blocking VEGF may prevent the growth of new blood vessels, including normal blood vessels and blood vessels that feed tumors. Unlike chemotherapy that attacks the cancer cells, the purpose of Avastin is to block the blood supply that feeds the tumor. This can stop the tumor from growing. (Genentech)
Avastin is currently used in two distinct ways for the treatment of RRP.
- Intralesional: Injected directly into the diseased site, either in the OR under general, or during an in-office treatment for RRP.
- Systemic: Administered via an IV, most often in an oncology infusion center.
Which delivery method is best for myself or my child?
The RRP Patient Registry currently reports intralesional Avastin use is seen in patients regardless of aggressiveness of disease; whereas, systemic (IV) use of Avastin is reserved for those patients with aggressive disease and/or pulmonary disease. (See consensus statement below)
- High blood pressure
- Too much protein in the urine
- Nosebleeds
- Bleeding
- Back pain
- Headache
- Taste change
- Dry skin
- Inflammation of the skin
- Inflammation of the nose
- Watery eyes
Important steps if pursuing systemic care?
The RRPF recommends you request your care team reach out to one of the top teams using this therapy on patients. We are happy to connect your team with those MD’s/Researchers. This is likely to bring you a better/safer outcome.
Current consensus statement on the use of “Systemic Avastin”:
The RRPF recommends that if you are considering this therapy, that your team follows the work on dosing and schedule of Dr. Hartnick, Dr. Zeitels et al at Mass General. Your clinician should have their contact information; if not we can assist you with that. Their work on this therapy, including suggestions on dosing/scheduling.
Conclusions and relevance: Intralesional bevacizumab treatment may increase duration of time between surgical procedures and decrease number of procedures per year, while improving voice QOL.